Pictures, Photos, Images
Descriptions, & Reviews.
Honey Bee Diseases & Pests. Page Four
George & Eve DeLange
We Are Proud Of Our SafeSurf Rating!
Honey bees (or honeybees) - Diseases & Pests:
We wish to thank Wikipedia, the free encyclopedia for some of the information on this page. We share images and information with Wikipedia. We also wish to thank the Biosecurity Sciences Laboratory in Archerfield, BC for their free information and images that they provide to help people interested in bee pests and diseases.
Biosecurity Sciences Laboratory
Most Honey Bees of the world can protect themselves pretty well. The queen has the ability to sting repeatadly but the worker bees can only sting once and they lose their stinger as it pulls out of their body after they sting. The drone bees have no stinger and can not sting at all. Most bees are gentle and will resist stinging unless they think it is down to a life or death situation to protect the hive. The African bee is the exception to this general rule. That is why it is such a dangerous species. Unfortunately the honey bee has many enemies and this page will show some of the more common enemies and diseases. But, first, I want to discuss what you should do if you get stung by a honeybee.
What's the best way to remove a bee stinger?
There's some controversy surrounding the best method for removing a bee stinger. Some say it needs to be scraped out to avoid squeezing more venom into the skin. This is the theory, I was taught, and agree with. Others say; "just grab it and pull it out." "Just pull it out, brush it off, or scrape it off." "Just get it out fast!" "What really matters is how quickly you remove it." There's no scientific evidence that one way of removing a bee stinger is better than another. Now, with that being said! I always scrape it off, as fast as I can! I was taught that scraping it off, keeps more poison from entering the wound. And from my own personal experience; the faster you scrape it off, the better! Now, let's take a look at some of the more common enemies, and diseases of the honeybee.
Chalkbrood, Ascosphaera apis.
Chalkbrood is caused by a fungus called Ascosphaera apis; which infests the gut of the larva. The fungus will compete with the larva for food, ultimately causing it to starve to death. The fungus will then go on to consume the rest of the larva's body, causing it to appear white and 'chalky'. Thus, the common name, Chalkbrood. The fungual spores are highly infectious and are they are carried in contaminated pollen by infected foraging bees. This leaves spores at floral and water sites, by queens, drifting bees, and drones. Shifting bees on trucks with an open entrance causes drift and hence spreads disease. The spores remain viable for up to 15 years or more in equipment and soil. Use of contaminated sites and old equipment could lead to infections. Interchange of equipment by the beekeepers also spreads the disease. Diseased larvae can usually be found throughout the brood-rearing season, but they are most prevalent in late spring when the broodnest is rapidly expanding. Usually the affected larvae are found on the outer fringes of the brood nest where there are insufficient nurse bees available to maintain an elevated brood nest temperature. The brood cells can be either sealed or unsealed. Young pupae or recently sealed larvae are most susceptible. The house bees often puncture or remove cappings. Sometimes, you will find dead larvae or pupa in front of the hive, where the house bees have removed them. Chalkbrood is most commonly visible during wet spring times in most areas of the world. It is not a "big" problem in Arizona; but it can happen! There are no registered chemicals available for chalkbrood control, the only means of controlling the disease is through management practices and use of disease-resistant bees. Hives with Chalkbrood Disease can generally be saved by increasing the ventilation throughout the hive. Maintaining strong healthy colonies has been demonstrated to reduce the effects of chalkbrood.
Management practices which may reduce the effects of chalkbrood disease are:
Greater & Lesser Wax Moths, Galleria mellonella, & Achroia grisella.
There are two species of Wax Moths - the greater wax moth, Galleria mellonella, and the lesser wax moth, Achroia grisella. The Greater Wax Moth or Honeycomb Moth (Galleria mellonella) is a moth of the family Pyralidae. It is the only member of the genus Galleria. It is found in most of the world, including Europe and adjacent Eurasia (its presumed native range), and as an introduced species e.g. in North America and Australia. Achroia grisella, the Lesser Wax Moth, is a small moth of the snout moth family (Pyralidae), wherein it belongs to the subfamily Galleriinae. It is the only unequivocally recognized species of genus Achroia. The Greater Wax Moth (Galleria mellonella) is a quite close relative; though placed in a distinct genus, it still belongs to the same tribe � the Galleriini � within the Galleriinae. Their caterpillars larvae are among the best-known "waxworms", as they are widely bred as food for pets such as lizards or fish. The larvae are the only ones that eat, the adults will not eat. Their diet typically consists of honey, beeswax, stored pollen, bee shell casings, and in some cases bee brood. Their larvae tunneling through honeycombs; not only provides food for the larvae, but also protects the larvae from the defending worker bees. Maintaining strong healthy colonies has been demonstrated to reduce the problem of waxworms. They usually "attack" weak hives or diseased hives. There are no longer any recommended chemical methods to control waxworms. An older method was to use Moth Crystals or Moth Balls, but that leaves a poison residue on honeycomb and is not recommended. Temperature manipulation and carbon dioxide fumigation are forms of physical control that are recommended for rapid, safe, and effective wax moth protection. These control measures can be used for stored extracted comb or hive products intended for human consumption, such as comb honey or pollen. Cold Treatment: minimum cold temperature storage time required to kill all life stages of wax moths in honey-extracted comb include: 20�F (-7�C) for 4.5 hours, 10�F (-12�C) for three hours, or 5�F (-15�C) for two hours. Additional time should be given for equipment to reach required minimum temperatures, especially in hot weather or large capacity freezers. These temperature exposure periods will have to be increased to kill wax moth larvae in comb honey. Wax moth development is accelerated at higher temperatures, so comb honey should be protected from this pest beginning immediately after harvest. Freezing individual frames containing wax moth larvae from live bee colonies is recommended to control this pest, but this will rarely result in successfully salvaging a colony that also shows signs of weakness and low morale. Some other more serious primary problem such as queen failure, mites, or disease is responsible for the poor condition of the colony. Heat Treatment: heat can be used to kill all life stages of wax moths by using the following exposure periods: 115�F (46�C) for 80 minutes or 120�F (49�C) for 40 minutes. Treatment exposure periods should not begin till specified temperatures are reached. Combs should not be heated above 120�F (49�C) because combs will sag above this temperature and beeswax melts at about 148�F (64�C). Frames of comb should be heat-treated only in the upright position and should not be handled until allowed to cool. Heat treatment should be used only for comb containing little or no honey (Shimanuki and Knox 1997). Carbon Dioxide Treatment: carbon dioxide can be used as a fumigant to control wax moths in stored comb or comb honey. Air-tight treatment rooms or fumigation chambers are required to hold 80-98% carbon dioxide levels which have to be maintained continuously for up to 5 days at the lower levels to kill all life stages of wax moths. At the highest level (98% carbon dioxide) with a temperature of 100�F and relative humidity of 50%, only 4 hours are required to kill all life stages of the wax moth. Precaution: although no harmful carbon dioxide residues are left behind on treated comb or inside the fumigation chamber following use, a fully charged carbon dioxide room is hazardous to humans and can result in death.
Sacbrood, Sacbrood virus (SBV), Morator aetatulas.
Morator aetatulas is the virus that causes sacbrood disease. Affected larvae change from pearly white to a yellowish colour, then grey, then brown and finally from dark brown to mostly black. Death occurs when the larvae are upright, just before pupation. Consequently, affected larvae are usually found in capped cells. Usually worker larvae, and pupae; of seven to ten days of age, are the only ones affected, though sometimes drones may also become affected. The nurse bees transmit the virus when they feed larvae with brood food from their hypopharyngeal glands. Head development of diseased larvae is typically retarded. The head region is usually darker than the rest of the body and may lean toward the center of the cell. When affected larvae are carefully removed from their cells, they appear to be a sac filled with water. Typically the scales are brittle but easy to remove. Sacbrood-diseased larvae have no characteristic odor. Sacbrood is rarely considered a threat by beekeepers, however recent estimates suggest that one larva killed by the sacbrood virus contains enough virus to kill over one million healthy larvae in a hive. When looking at a brood comb, taken from the hive; symptoms of sacbrood are partially uncapped cells scattered about the frame or capped cells that remain sealed after others have emerged. The diseased individuals inside cells will have characteristically darkened heads which curl upward. The dead prepupa resembles a slipper inside the cell. Diseased prepupae fail to pupate and turn from pearl white to pale yellow to light brown and finally, dark brown. The skin is flaccid and the body watery. The dark brown individual becomes a wrinkled, brittle scale that is easily removed from the cells (unlike AFB). Usually there is no odor, although in advanced stages, an occasional putrid smell may be detected. The house bees, which are contaminated while removing infected dead brood, probably spread the virus. Serious outbreaks of sacbrood are rare and control measures are usually not necessary; because adult bees normally detect and quickly remove the infected larvae. If control does become necessary, strengthening of the colony and requeening are suggested.
European Foulbrood, Melissococcus plutonius.
Melissococcus plutonius is a bacterium that causes European Foulbrood. Melissococcus plutonius is a bacterium that infests the mid-gut of an infected bee larva. European foulbrood is less deadly to a colony than another common bacterial disease called, American foulbrood. Melissococcus plutonius does not form spores, although it can overwinter on honeycomb. Symptoms of European Foulbrood include dead and dying larvae which can appear curled upwards, brown or yellow, melted or deflated with tracheal tubes more apparent, and/or dried out and rubbery. European Foulbrood is often considered to be caused by "stress." A bacterial disease that is dangerous or deadly, only if the colony is already under stress for other reasons. It is said that an otherwise healthy colony can usually survive European foulbrood. Outbreaks of European Foulbrood may be controlled chemically with oxytetracycline hydrochloride, but honey from treated colonies probably would have chemical residues from the the antibiotic treatment.
American Foulbrood, Paenibacillus larvae ssp. larvae.
American foulbrood (AFB), caused by the spore- forming Paenibacillus larvae ssp. larvae (formerly classified as Bacillus larvae). American foulbrood is the most widespread and destructive of all of the bee brood diseases. The Paenibacillus larvae is a rod-shaped bacterium, which is visible only under a very high power microscope. The larvae up to 3 days old become infected by ingesting spores that are present in their food. The young larvae, less than 24 hours old are most susceptible to infection. Spores germinate in the gut of the larva and the vegetative form of the bacteria begins to grow, taking its nourishment from the larva. Spores will not germinate in larvae over 3 days old. Infected larvae normally die after their cell is sealed. The vegetative form of the bacterium will die but not before it produces many millions of spores. Each dead larva may contain as many as 100 million spores. This disease only affects the bee larvae but is highly infectious and deadly to bee brood. Infected larvae darken and die. Because of the persistence of the spores (which can survive up to 40 years), many State Apiary Inspectors require an AFB diseased hive to be burned completely. American foulbrood spores are extremely resistant to desiccation and can remain viable for more than 40 years in honey and beekeeping equipment. Therefore honey from an unknown source should never be used as bee feed, and used beekeeping equipment should be assumed contaminated. Until about 1906, European Foulbrood, & American Foulbrood; were not differentiated; and their conditions were generally referred to as just Foulbrood.
Honey Bee Tracheal Mite, Acarapis woodi.
The Honey Bee Tracheal Mite, Acarapis woodi is a mite, that is an internal parasite of honey bees. Tracheal mites are related to spiders and have eight legs. Acarapis woodi live and reproduce in the tracheae of the bees. The female mite attaches 5 � 7 eggs to the tracheal walls, where the larvae hatch and develop in about 11�15 days into adult mites. The Tracheal Mites parasitize young bees up to two weeks old through the tracheal tube openings. There they pierce the tracheal tube walls with their mouthparts and feed on the haemolymph of the bees. More than a hundred mites can populate the tracheae and weaken the bees. The mites are generally less than 175 micrometres (0.007 inch) long, and can only be seen and identified under a microscope Other mites that are similar in appearance are the Acarapis externus, & Acarapis dorsalis.
Varroa Mite, Varroa destructor,
Varroa destructor is an external parasitic mite that attacks honey bees Apis cerana and Apis mellifera. The honeybee disease caused by the mites is called varroatosis. The Varroa are a genus of parasitic mites associated with honey bees, placed into their own family, the Varroidae. The genus was named for Marcus Terentius Varro, a Roman scholar, who was also a beekeeper. Varroa jacobsoni is thought to be the species causing the problem, but new research shows that Varroa jacobsoni is a species complex containing 18 different genetic variants that belong to 2, possibly 5 different species of Varroa. Researchers, Anderson and Trueman have indicated that they were unable to find morphological differences to distinguish between the genetic types. Varroa mites were first reported in the Bluegrass region of Kentucky in 1991. They have now spread to and become a major pest of honey bees in almost all states of the USA. These external honeybee parasites, attack both the adults and the brood of honeybees, with a distinct preference for drone brood. They suck the hemolymph (blood) from both the adults and the developing brood, weakening and shortening the life span of the ones on which they feed. By sucking the hemolymph, RNA viruses, such as the deformed wing virus (DWV) spreads to the bees. The emerging brood may be deformed with missing legs or wings. Untreated infestations of varroa mites that are allowed to increase will definately kill honeybee colonies, usually in the late autumn through early spring. Losses due to these parasitic mites are often confused with other causes; such as, winter mortality and queenlessness, if the colonies are not examined for Varroa Mites. The Varroa Mite is the parasite with the most pronounced economic impact on the beekeeping industry today. It may be a contributing factor to the Colony Collapse Disorder (CCD). The adult female mites are reddish-brown in color, have a flat, button shape; they are 1 � 1.8 mm long and 1.5 � 2 mm wide; and they have eight legs. They are large enough to be seen with the unaided eye on the thorax, most commonly, and on the bee's abdomen. Their flattened shape allows them to hide between the bee's abdominal segments. This mite is often confused with the bee louse, but the bee louse has only six legs, is more circular in shape, and is slightly larger. Varroa Mites develop on the bee brood. A female mite will enter the brood cell about one day before capping and be sealed in with the larva. Eggs are laid and mite feed and develop on the maturing bee larva. By the time the adult bee emerges from the cell, several of the mites will have reached adulthood, mated, and are ready to begin searching for other bees or larvae to parasitize. There is a preference for drone brood. Inspection of the drone brood in their capped cells will often indicate whether or not a colony is infested. The dark mites are easily seen on the white pupae when the comb is broken or the pupae are pulled from their cells. Varroa Mites reproduce on a 10-day cycle. First the female mite enters a honey bee brood cell. Then as soon as the cell is capped, the Varroa Mite lays 2-6 eggs at approximately 30-hour intervals, on the larva which hatch into several females and typically one male. Then, the young mites hatch at about the same time as the young bee develops and leaves the cell with the host. While all of this is happening, The mites development proceeds from egg to six-legged larvae, to eight-legged protonymphs, to deutonymphs, to sexually mature adult mites in 6 to 10 days. They mate in the capped cells with the males dying soon afterward. All immature mites will die after the emerging bee opens the cell, while the young adult female mites and the mature (gravid) females move on to passing bees. The mite enters another brood cell in 3 to more than 150 days depending on the season and availability of brood. So, when the young bee emerges from the cell after pupation the Varroa Mites also leave, and spread to the other bees and larvae. The Varroa Mite prefers to infest drone cells. Mites spread from colony to colony by drifting workers and drones within an apiary. Honey bees can also acquire these mites when robbing smaller colonies. It is best to isolate captured swarms, package bees, and other new colonies from other colonies and examine them for mites before placing them in an apiary. How To Control Varroa destructor Infections. Note: Early detection of low levels of mite infestations is the key to its successful management. Every time you go into the hive, look for them! If you find Varroa Mites in any hive, then ll colonies at the site should be treated for Varroa Mites. One product is available that will kill the mites and cause the mites to drop from the bees. But, Varroa Mites are developing a resistance to it! It is called Apistan. If you use it, two strips should be hung in the brood nest area of the colony for approximately 4 weeks. Apistan strips are to be used with sticky paper and a fine-mesh screen on the bottom board of a colony to capture any mites that may have been present. A considerable amount of cell cappings and other debris will also collect on the sticky paper, so you should inspect the sticky paper carefully for any Varroa Mites after removal of the strips, & sticky paper. Apistan strips are available from most large beekeeping suppliers and they can be used for the detection and treatment of Varroa infestations. Important Note: Apistan strips contain the miticide fluvalinate and NEVER should be used during the honey flow, or when there is surplus honey present in the colony that may be removed for consumption. It is said that in the late fall, after removal of surplus honey; or in the early spring, prior to the honey flow, are the best times to treat for Varroa Mites. But remember, Varroa Mites are developing a resistance Apistan!
Manley's Thymol Crystal and surgical spirit: Manley's Thymol Crystal and surgical spirit recipe with sugar as food. Essential oils: Essential oils, especially lemon, mint and thyme oil. Sugar esters (Sucrocide): Sugar esters (Sucrocide) in spray application. Oxalic acid: Oxalic acid trickling method or applied as vapor. Formic acid: Formic acid as vapor or pads. Foodgrade mineral oil: Foodgrade mineral oil as vapor and in direct application on paper or cords.
The screened bottom board is also being credited with increased circulation of air which reduces condensation in a hive during the winter. However, studies at Cornell University, done over a two year investigation; found that screened bottoms have no measurable effect! Screened bottom board with sticky board: It separates mites that fall through the screen and the sticky board prevents them from crawling back up. Small cell foundation: Small cell foundation (4.9 mm across�about 0.5 mm smaller than standard) is believed to limit the space in each cell that Varroa mites have in which to inhabit and also to enhance the difference in size between worker and drone brood with the intention of making the drone comb traps more effective in trapping Varroa mites. Small cell foundation has staunch advocates though controlled studies have been generally inconclusive. Comb trapping: Comb trapping is an advanced method that removes capped brood from the hive, where the Varroa mites breed. The queen is confined to comb A using a comb cage. After nine days the queen is confined to a new comb�comb B�and the brood in comb A is left to be reared. Nine days later the brood in comb A�now capped and infested with Varroa mites�is removed. The queen is then removed from comb B and placed on to comb C, with the brood in comb B left to be reared. Nine days later comb B is removed and the queen is excluded from comb B. Nine days later comb C is removed. This complex method can remove up to 80% of Varroa mites in the hive. Powdered sugar (Dowda Method): Powdered sugar (Dowda Method), talc or other "safe" powders with a grain size between 5 and 15 micrometres can be sprinkled on the bees. The powder does not harm the bees (and, in the case of sugar, can even become a small food source), but does interfere with the mite's ability to maintain its hold on the bee. It is also believed to increase the bees' grooming behavior. This causes a certain percentage of mites to become dislodged. Powdered sugar works best with a screened bottom board.
There are other pests and diseases of honeybees, but these that we have discussed, are the most common.
|
 |  |
| Most Of The Time Bees Won't Bother You | But, Sometimes They Leave You A Present!! |
|---|---|
 |  |
| Always Scratch The Stinger Out If You Get Stung. Don't Pull It Out! | Pulling It Out Squirts Poison In! It Didn't Take The Bees Long To Find This Tender Spot!! |
 |  |
| Normal Healthy Brood | Chalk Brood (A Fungus Disease) |
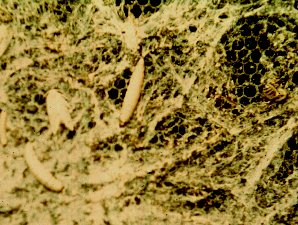 |  |
| The Wax Worm And Moth Probably Destroys More Hives Than Anything | Actually The Wax Moth Cleans Up Sick And Weak Bee Hives By Eating Up The Wax And Honey |
 |  |
| The Greater Wax Moth. Galleria mellonella. Dorsal View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | The Greater Wax Moth. Galleria mellonella. Ventral View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
 |  |
| The Greater Wax Moth. Galleria mellonella. Dorsal View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | The Greater Wax Moth. Galleria mellonella. Cocoon. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
 |  |
| The Lesser Wax Moth. Achroia grisella. Dorsal View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | The Lesser Wax Moth. Achroia grisella. Ventral View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
 |  |
| The Lesser Wax Moth. Achroia grisella. Dorsal View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | The Lesser Wax Moth. Achroia grisella. Cocoons. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
 |  |
| The Lesser Wax Moth. Achroia grisella. Larvae. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | The Lesser Wax Moth. Achroia grisella. Larvae. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
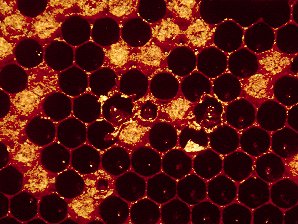 |  |
| Sacbrood, Caused By: Sacbrood virus (SBV), Morator aetatulas. | European Foul Brood Showing Flat Capping, Sunk Capping, Dead Larvae |
 |  |
| European Foul Brood Showing Dead Larvae And Scales | American Foul Brood Showing Tongue Attachment |
 |  |
| American Foul Brood Showing Flat Cappings, Dead larvae | American Foul Brood Showing Ropiness Of Dead Larva |
 | |
| Crop Dusters Kill Bees | The Rouge Shooter |
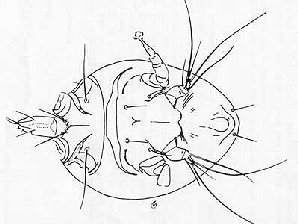 | 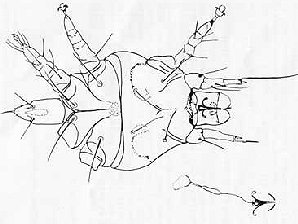 |
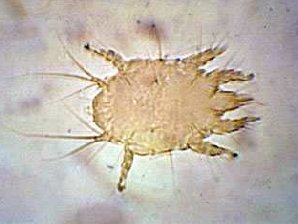
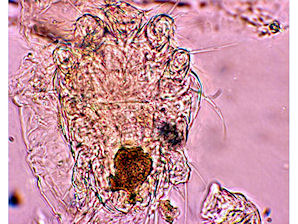
| Honey Bee Tracheal Mite Female ( Acarapis woodi ) | Honey Bee Tracheal Mite Male ( Acarapis woodi ) |
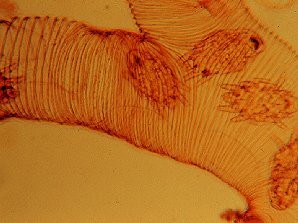 |  |
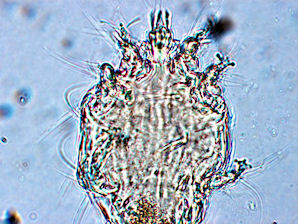
| Tracheal Mites In Bee Trachea | Tracheal Mite |
 |  |
| Tracheal Mite. Acarapis woodi. Female Mouthparts. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | Tracheal Mite. Acarapis woodi. Male With Food Meal. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
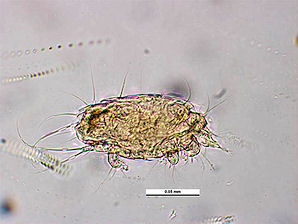 | 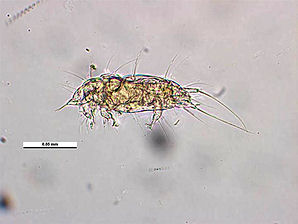 |
| Tracheal Mite. Acarapis woodi. Dorsal View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | Tracheal Mite. Acarapis woodi. Lateral View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
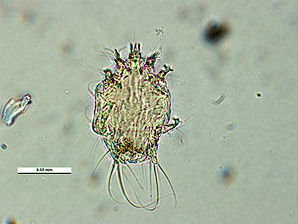 | 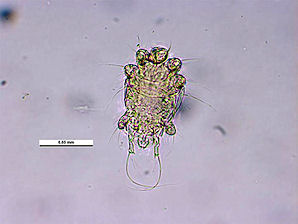 |
| Tracheal Mite. Acarapis woodi. Venteral Female View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | Tracheal Mite. Acarapis woodi. Venteral Male View. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
 | 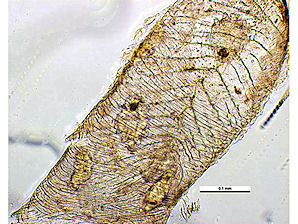 |
| Tracheal Mites In Bee Trachea. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. | Tracheal Mites In Bee Trachea. Courtesy: Museum Victoria Simon Hinkley & Ken Walker. |
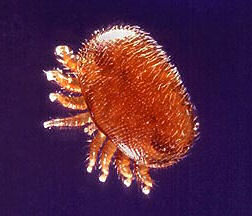 |  |
| Varroa destructor, Mite. Courtesy: Wikipedia, the free encyclopedia. | Varroa destructor, Mite. Courtesy: Wikipedia, the free encyclopedia. |
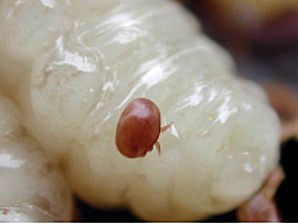 | 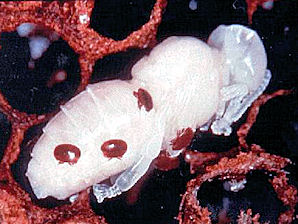 |
| Varroa destructor, Mite. Having Sucked "Blood" From Bee Larvae. Courtesy: Wikipedia, the free encyclopedia. | Varroa destructor, Mite. Having Sucked "Blood" From Bee Pupa. Courtesy: Wikipedia, the free encyclopedia. |
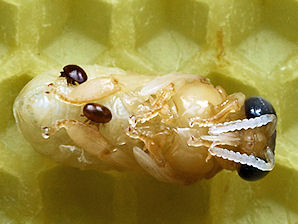 |  |
| Varroa destructor, Mite. Having Sucked "Blood" From Bee Pupa. Courtesy: Wikipedia, the free encyclopedia. | Varroa destructor, Mite. Having Sucked "Blood" From Bee Pupa. Courtesy: Wikipedia, the free encyclopedia. |
Here Are Some Links To The Very Best & Most Popular Items Sold On Amazon.Com
To Learn More! Click The Links Below. No Obligation, Of Course!
